The authoritative web-based application for creating, publishing, and managing Health Product Declarations (HPDs) in full compliance with HPD Open Standard Version 3.0.
What's New in HPD Builder
HPD Open Standard Version 3.0 is now the effective version as of September 29, 2025
Version 3.0 Now Live
On September 29th, 2025, the Health Product Declaration Collaborative (HPDC) released HPD Open Standard Version 3.0. The HPD Builder now ensures full conformance to this updated standard, with all newly published HPDs using Version 3.0.
Download the Standard
HPD Format Update
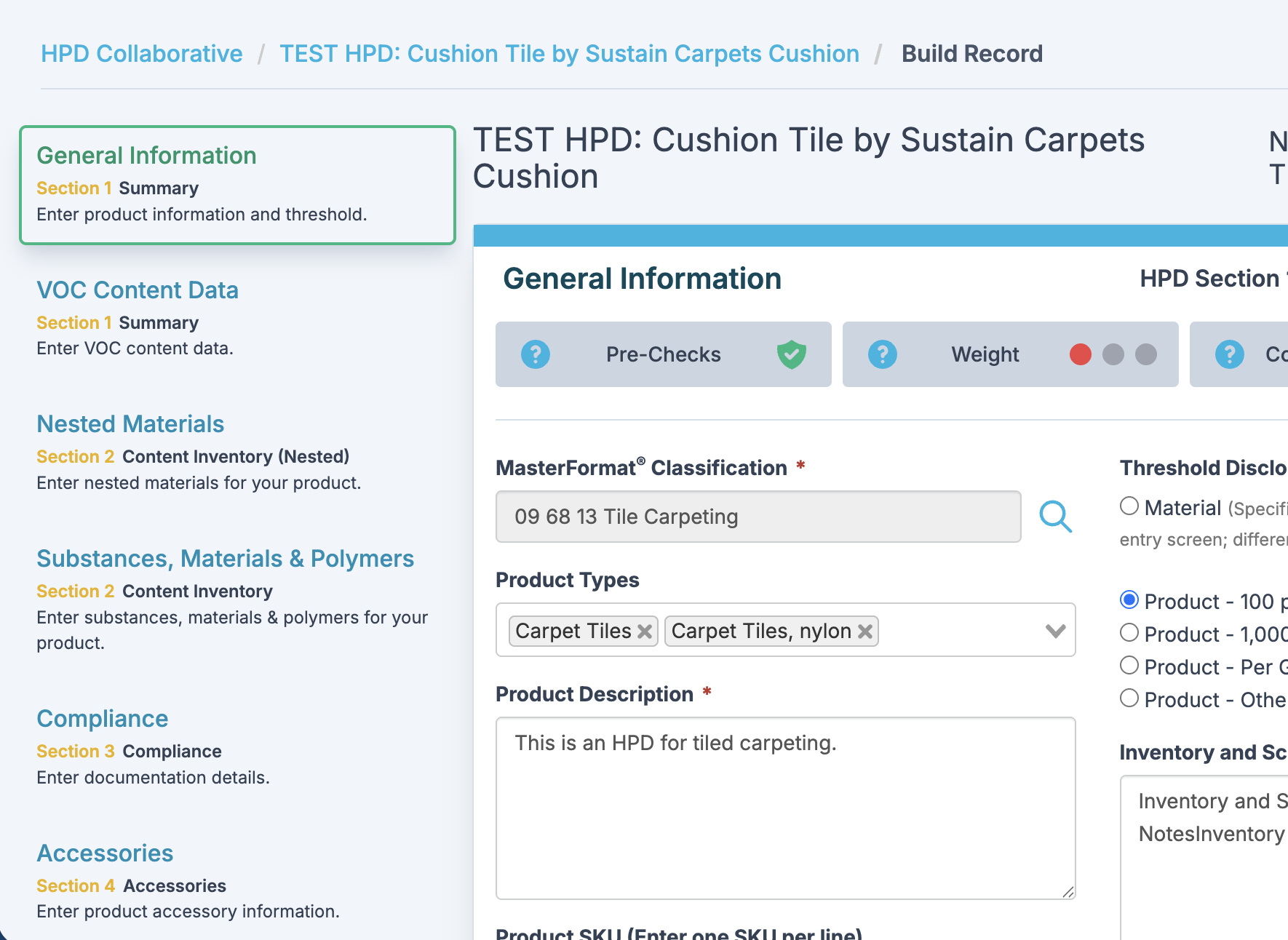
Simplified data display on HPD format Summary for improved readability and user experience.
Product Type Taxonomy
More granular classification system improves product searchability and filtering, aligned with industry standards.
Unique Product ID
Accurate and unique identification for transparency in material health reporting and compliance tracking.
EC Number Screening
Pharos screening now supports EC Numbers, improving compatibility with European chemical regulations.
Metal Alloy Inventory
New Alloying Element Inventory allows detailed reporting of metal compositions with individual hazard screening.
PFAS Attestation
Manufacturers can declare PFAS presence below reporting thresholds with built-in screening tool integration.
Need Help with HPD v3.0?
For questions and technical assistance using HPD v3.0 and HPD Builder, visit our support portal.
Visit Support PortalWhat is HPD Builder?
A web-based application to create, publish and manage HPDs
Designed to support full compliance with HPD Open Standard Version 3.0 requirements and capabilities
Uses Habitable Pharos Chemical Material Library (CML) to provide reliable substance and hazard information
Authoritative method for "publishing" HPDs to the HPD Public Repository

Major Benefits and Features
Assists manufacturers in preparing HPDs compliant with HPD Open Standard 3.0
Reduces data entry duplication and errors
Simplifies HPD creation and publishing processes
Enables permission-based access to manufacturers HPD by suppliers and consultants
Includes provision for confidential business information, with option to disclose Hazards without identifying substance name or CASRN
Provides automated data exchange with 3E Exchange
Trusted by Industry Leaders
Join thousands of manufacturers creating transparent, compliant HPDs
Ready to Get Started?
Create your first Health Product Declaration today and join the movement toward transparent, healthy building materials.
© 2026, Health Product Declaration Collaborative. All Rights Reserved.